Sugiyama, Akira

Sugiyama, Akira
Assistant Professor
Radiation Worker
- https://www.ric.u-tokyo.ac.jp/article/sugiyama.html
- https://www.u-tokyo.ac.jp/focus/ja/people/people100043.html
- https://researchmap.jp/lisabio?lang=en
Areas of expertise
Radiopharmaceutical development, Radiation Effects Science
Description of your research
DEVELOPMENT OF NEXT-GENERATION TARGETED RADIOPHARMACEUTICALS PLATFORM
Development of Technology to Deliver Radionuclides to Targets
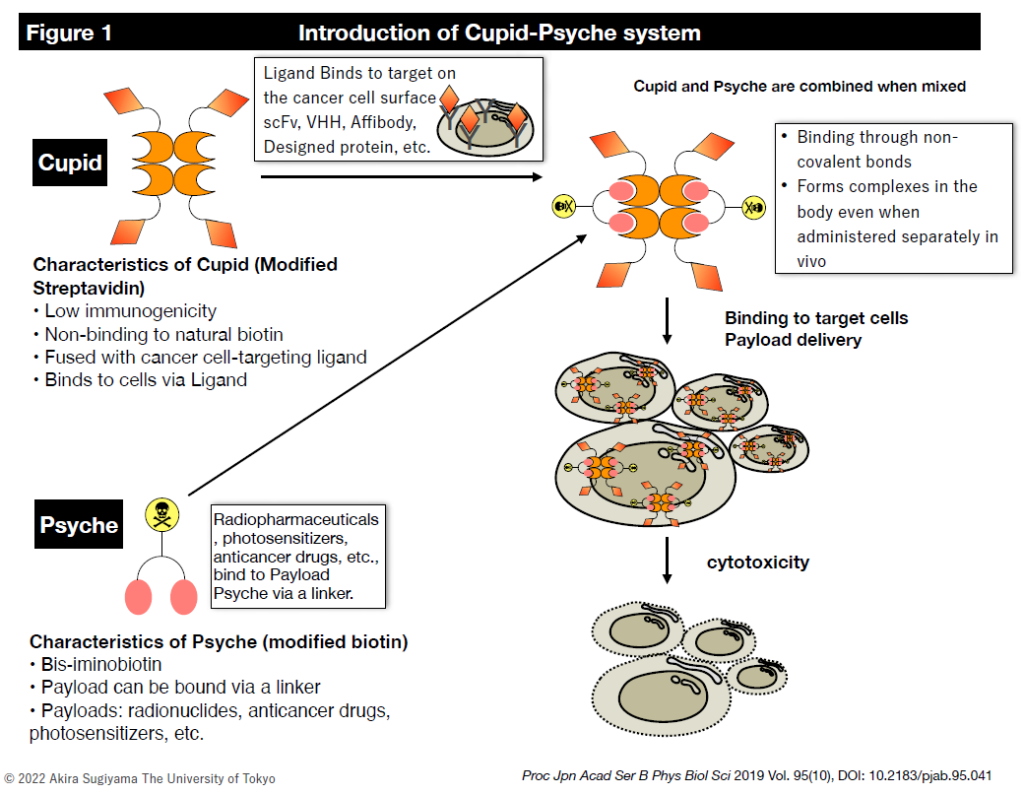
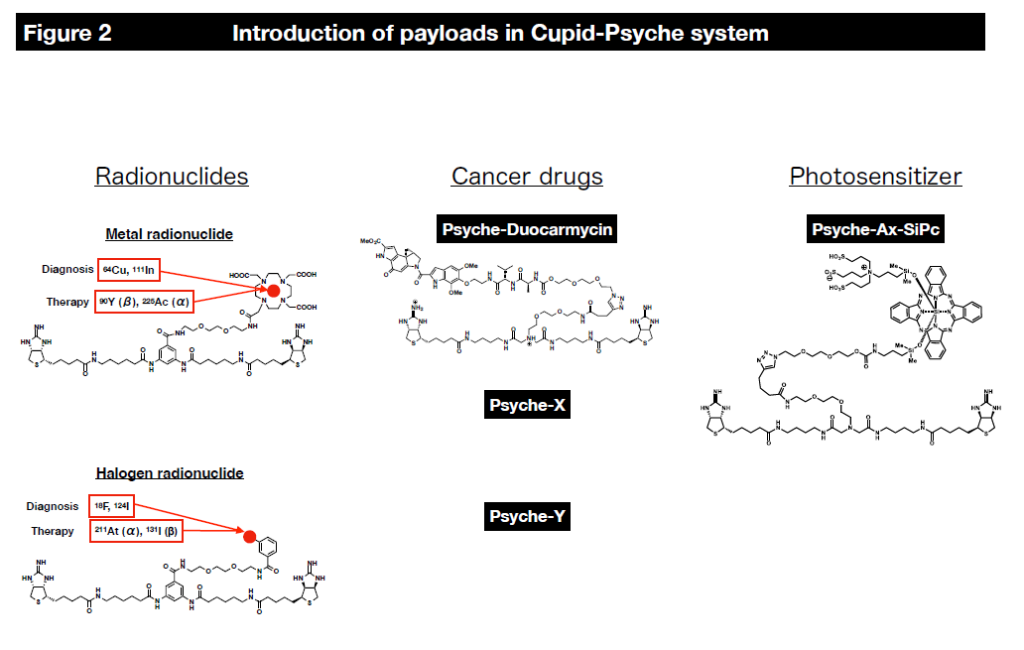
We are developing next-generation antibody-drug conjugates that can treat numerous molecular targets with various drugs (Figure 1). Currently, the drugs (payloads) that can be delivered include radionuclides (diagnostic and therapeutic), photosensitizers, and anticancer drugs (Figure 2).
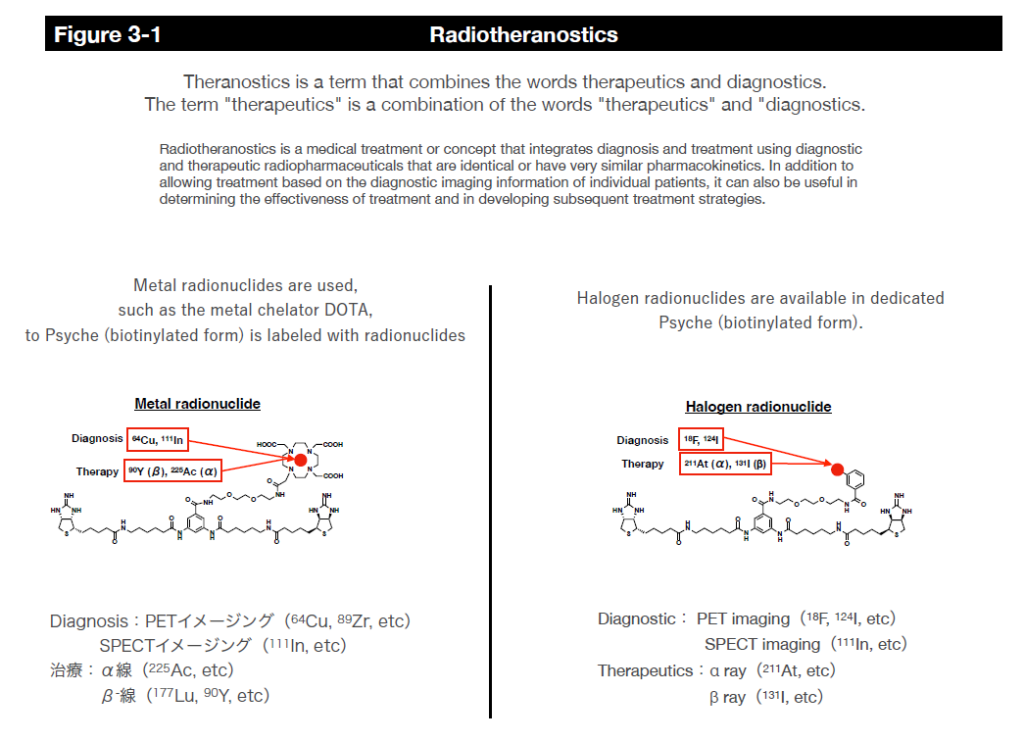
In this system, radionuclides can be delivered to cancer cells using a chelating agent called DOTA as a payload, including diagnostic radionuclides such as 111In (for SPECT) and 64Cu (for PET) and therapeutic radionuclides such as 90Y (β-ray), 177Lu (β-ray) and 225Ac (α-ray). It is also possible to label and deliver halogens such as 211At (α-ray) to cancer cells using other binding modes.
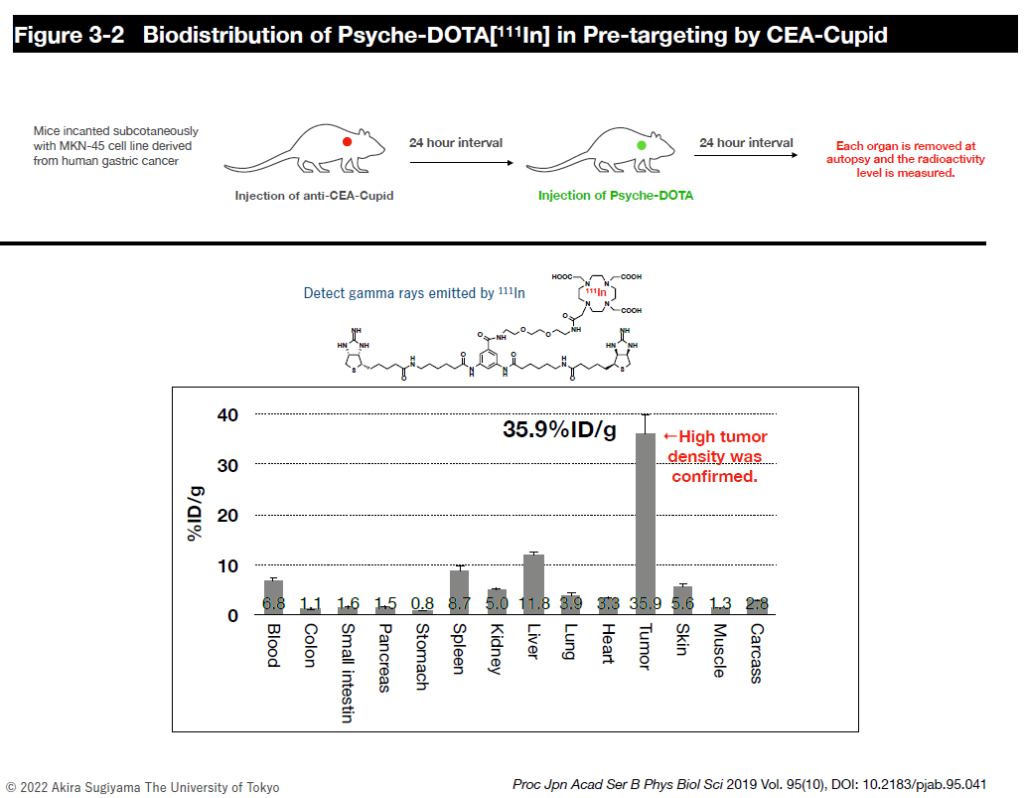
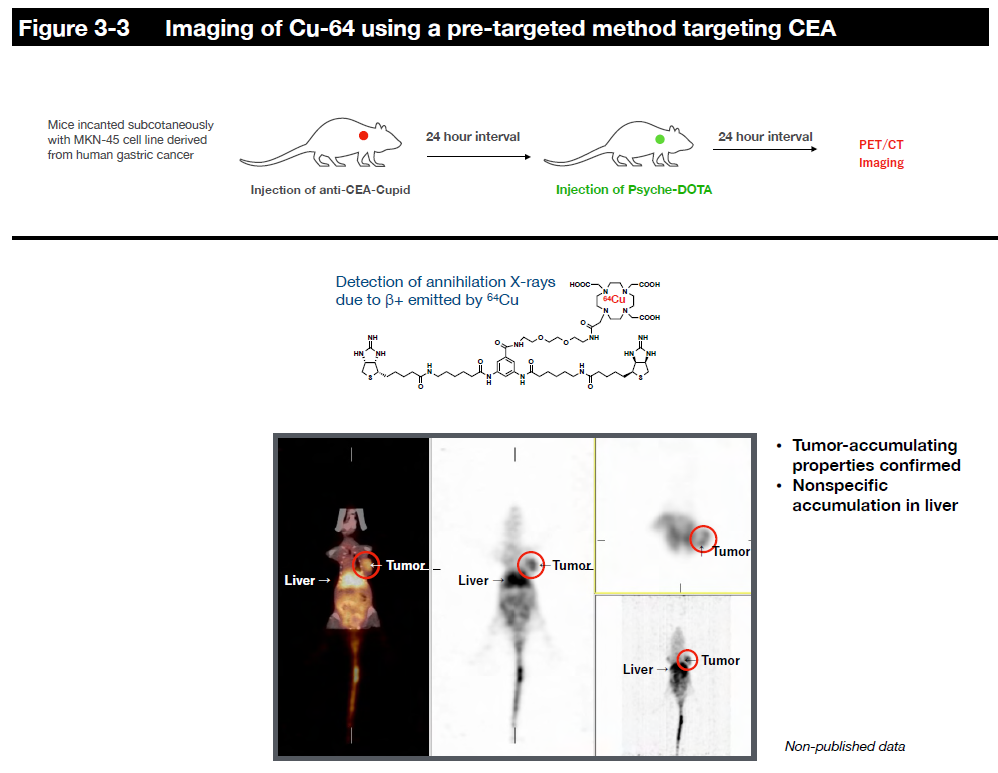
However, since radiopharmaceuticals consistently emit radiation and radiation on/off cannot be controlled, we are collaborating with researchers at the Faculty of Pharmaceutical Sciences and Fukushima Medical University to establish a delivery method called the pre-targeting method to reduce side effects on normal tissues as much as possible. In this joint research, we are designing and synthesizing payload molecules that have a kinetic behavior in the body so that radiopharmaceuticals accumulate quickly at the target site without circulating in the body for a long time, and those that are not bound to the radiopharmaceuticals are excreted rapidly out of the body. We are also researching and developing the practical use of internal therapy for alpha-ray emitting radionuclides, which have particularly strong cytotoxicity, while obtaining experimental data in mice.
Research and Development of Next-Generation Binding Agents
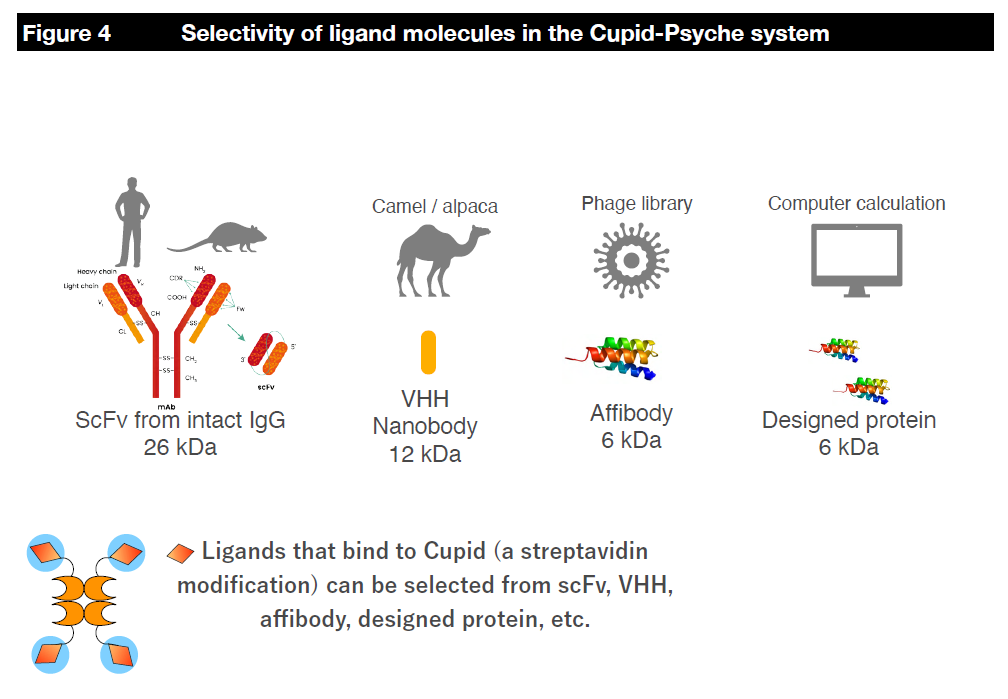
On the other hand, we are also designing and synthesizing binding agents (protein molecules) that bind to target molecules on the surface of cancer cells and deliver their payloads. For ligands that bind to target molecules, we have used single-chain variable fragment (scFv) antibodies produced from human and mouse IgG antibodies and single-domain antibodies (VHH, nanobody) derived from alpaca and antibody mimetics (affibody). We have experience in this area (Figure X). Recently, we have collaborated with molecular dynamics simulation researchers to design ligand molecules by computational methods. Although scFv (26kD), which has a significant molecular weight, was problematic for mass production, we developed a way to rapidly produce the target protein in E. coli by using VHH (12kDa) and affibody (6kDa), which have a small molecular weight (Reference DOI:10.1016/j.pep.2021.106043). This enables us to immediately manufacture the computationally designed molecule as a conjugate and evaluate its activity. We are developing a new conjugate manufacturing platform that combines AI and computational design.
Platform technology for new therapeutic targets and new radionuclides
Therapeutic nuclides are being researched and developed worldwide, with a shift from 90Y to 177Lu, which has a higher response rate, for β-ray nuclides, 211At and 225Ac for α-ray nuclides, and new therapeutic nuclides such as 212Pb.
In addition, radiation in vivo is being promoted for practical use in clinical diagnosis, treatment, and basic research. Specifically, in the "Medical Diagnosis of In Vivo Chemical Environment by Creation of Entangled Photon-Emitting Nuclei and Molecular Probes" in JSPS Grant-in-Aid for Transformative Research Areas (B), in which researchers from the School of Engineering at the University of Tokyo are serving as principal investigators, it has become possible to detect changes in the in vivo chemical environment caused by radiation emitted from radionuclides by systematizing detection theory and developing detection devices. Basic research on applying these technologies to medical care has been initiated.
We are conducting research and development to provide a target-targeting platform that meets a wide range of needs, including actual clinical diagnosis and treatment, as well as research on new bio-environmental information obtained from radiation emitted by radionuclides delivered into the body.
Competitive research funds related to this research
- FY 2014-15 AMED P-DIRECT
“Practical Application of Advanced Colorectal Cancer Pre-targeting Therapeutics Integrating PET Imaging Diagnostics and Isotope DDS Therapy" (Co-Principal Investigator) - FY 2016-17 AMED P-CREATE
“Development of an innovative cancer cell targeting system for the diagnosis and treatment of advanced metastatic cancer" (Co-Principal Investigator) - FY 2017-18 JSPS Bilateral Joint Research Project (JSPS-STINT (Japan-Sweden))
"Pre-targeted drug development using Astatin-211 for the treatment of metastatic cancer " (Co-Principal Investigator) - FY 2018-20 AMED Practical Research for Innovative Cancer Control
"Development of a Therapeutic Agent for Intractable Cancer by Internal Therapy with Alpha-Ray Emitting Nuclei " (Co-Principal Investigator) - FY 2018-20 Fundamental Research (B)
Development of robotic purification and labeling technology to reduce radiation exposure in short-lived α-ray pharmaceutical manufacturing processes" (Co-Principal Investigator) - FY 2019-21 Fundamental Research (B)
"Experimental study of internal irradiation therapy for peritoneal dissemination of gastric cancer using super-selective delivery short-lived α-rays" (Representative: Sachiyo Nomura) (Co-Principal Investigator) - FY 2020-22 Fundamental Research (B)
"Optimization of Pretargeting Methods for Targeted Alpha Therapy and Adaptation to Multiple Cancer Types" (Co-Principal Investigator) - FY 2020-22 Fundamental Research (B)
"Development of innovative leukemia therapy using alpha-ray emitting radionuclides targeting cancer stem cells" (Co-Principal Investigator) - FY 2021-23 Fundamental Research (B)
"Creation of Next-Generation Antibody Drug Conjugate Therapeutics for Advanced Cancer" (Principal Investigator) - FY 2022-24 JSPS Grant-in-Aid for Transformative Research Areas (B)
"Medical Diagnosis of In Vivo Chemical Environment by Creation of Entangled Photon Pair Emitting Nuclei and Molecular Probes" (Co-Principal Investigator) - FY 2023-27 Fundamental Research (A)
“Development of novel radiopharmaceuticals using α-ray emitters and antibody-based drug delivery systems” (Co-Principal Investigator)
List of publications on radiation research
1*, Yumura, K. et al. Mutations for decreasing the immunogenicity and maintaining the function of core streptavidin. Protein Sci 22, 213-221 (2013). https://doi.org:10.1002/pro.2203
2*, Kawato, T. et al. Crystal structure of streptavidin mutant with low immunogenicity. J Biosci Bioeng 119, 642-647 (2015). https://doi.org/10.1016/j.jbiosc.2014.10.025
3*, Kawato, T. et al. Structure-based design of a streptavidin mutant specific for an artificial biotin analogue. J Biochem 157, 467-475 (2015). https://doi.org/10.1093/jb/mvv004
4*, Kawato, T. et al. Structure-based design and synthesis of a bivalent iminobiotin analog showing strong affinity toward a low immunogenic streptavidin mutant. Biosci Biotechnol Biochem 79, 640-642 (2015). https://doi.org/10.1080/09168451.2014.991692
5, Tachibana, K. et al. Analysis of the subcellular localization of the human histone methyltransferase SETDB1. Biochem Biophys Res Commun 465, 725-731 (2015). https://doi.org:10.1016/j.bbrc.2015.08.065
6, Kado, Y. et al. Epiregulin Recognition Mechanisms by Anti-epiregulin Antibody 9E5: STRUCTURAL, FUNCTIONAL, AND MOLECULAR DYNAMICS SIMULATION ANALYSES. J Biol Chem 291, 2319-2330 (2016). https://doi.org/10.1074/jbc.M115.656009
7, Sugiyama, A. et al. Cupid and Psyche system for the diagnosis and treatment of advanced cancer. Proc Jpn Acad Ser B Phys Biol Sci 95, 602-611 (2019). https://doi.org/10.2183/pjab.95.041
8, Takahashi, K. et al. Axially Substituted Silicon Phthalocyanine Payloads for Antibody-Drug Conjugates. Synlett 32, 1098-1103 (2021). https://doi.org/10.1055/a-1503-6425
9, Kaneko, Y. et al. The serological diversity of serum IgG/IgA/IgM against SARS-CoV-2 nucleoprotein, spike, and receptor-binding domain and neutralizing antibodies in patients with COVID-19 in Japan. Health Sci Rep 5, e572 (2022). https://doi.org/10.1002/hsr2.572
10*, Kaneko, Y. et al. Pathological complete remission of relapsed tumor by photo-activating antibody-mimetic drug conjugate treatment. Cancer Sci 113, 4350-4362 (2022). https://doi.org/10.1111/cas.15565
11, Kobashi, Y. et al. Humoral immunity after second dose of BNT162b2 vaccine in Japanese communities: an observational cross-sectional study, Fukushima Vaccination Community Survey. Sci Rep 12, 18929 (2022). https://doi.org/10.1038/s41598-022-21797-x
12*, Yamatsugu, K. et al. Antibody mimetic drug conjugate manufactured by high-yield Escherichia coli expression and non-covalent binding system. Protein Expr Purif 192, 106043 (2022). https://doi.org/10.1016/j.pep.2021.106043
13, Furuya, G. et al. Nucleic acid-triggered tumoral immunity propagates pH-selective therapeutic antibodies through tumor-driven epitope spreading. Cancer Sci 114, 321-338 (2023). https://doi.org/10.1111/cas.15596
14, Kawashima, M. et al. Antibody and T-Cell Responses against SARS-CoV-2 after Booster Vaccination in Patients on Dialysis: A Prospective Observational Study. Vaccines 11, 260 (2023). http://dx.doi.org/10.3390/vaccines11020260
15, Tani, Y. et al. Varying Cellular Immune Response against SARS-CoV-2 after the Booster Vaccination: A Cohort Study from Fukushima Vaccination Community Survey, Japan. Vaccines 11, 920 (2023). http://dx.doi.org/10.3390/vaccines11050920
* Corresponding author

